A study of the influence of isotopic substitution on the melting point and temperature of maximum density of water by means of path integral simulations of rigid models
Abstract
The melting point of ice Ih, as well as the temperature of maximum density (TMD) in the liquid phase, has been computed using the path integral Monte Carlo method. Two new models are introduced, TIP4PQ_D2O and TIP4PQ_T2O, which are specifically designed to study D2O and T2O respectively. We have also used these models to study the “competing quantum effects” proposal of Habershon, Markland and Manolopoulos; the TIP4PQ/2005, TIP4PQ/2005 (D2O) and TIP4PQ/2005 (T2O) models are able to study the isotopic substitution of hydrogen for deuterium or tritium whilst constraining the geometry, while the TIP4PQ_D2O and TIP4PQ_T2O models, where the O–H bond lengths are progressively shortened, permit the study of the influence of geometry (and thus dipole moment) on the isotopic effects. For TIP4PQ_D2O–TIP4PQ/2005 we found a melting point shift of 4.9 K (experimentally the value is 3.68 K) and a TMD shift of 6 K (experimentally 7.2 K). For TIP4PQ_T2O–TIP4PQ/2005 we found a melting point shift of 5.2 K (experimentally the value is 4.49 K) and a TMD shift of 7 K (experimentally 9.4 K).
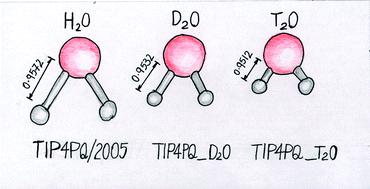

 Please wait while we load your content...
Please wait while we load your content...