The invalidity of the photo-induced electron transfer mechanism for fluorescein derivatives†
Abstract
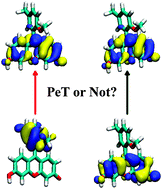
The ground and excited state geometries, the excitation and emission energies for a series of fluorescein derivatives in aqueous solutions have been investigated using time-dependent density functional theory (TD-DFT) with B3LYP and a long-range corrected CAM-B3LYP functional. The RI-CC2 method was employed to confirm the CAM-B3LYP results. As far as we know, the excited state geometries for a series of fluorescein derivatives were optimized for the first time, and the conformational changes upon photoexcitation were discussed. Importantly, the previous proposed photo induced

 Please wait while we load your content...
Please wait while we load your content...