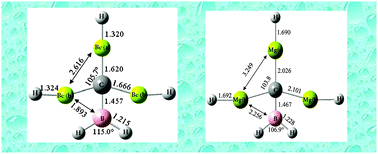
A class of neutral 18-electron molecules with planar tetracoordinate carbon (ptC) centers is introduced. We show computationally that when n = 3 the neutral singlet molecule C(BeH)n(BH2)4−n and other isoelectronic (18-valence electron) molecules of main group elements collapse from locally tetrahedral arrangements at the C-center to (near) planar tetracoordinate structures. For C(BeH)3BH2 and C(CH3)(BH2)Li2, for example, the tetrahedral type conformation is not even a minimum on the potential energy surface at the B3PW91, MP2(full), or CCSD levels of theory. The Mg analogue C(MgH)3BH2 of the Be system also features a completely flat global minimum (with even higher energy planar minima in both cases as well). Other neutral compounds that may prefer planar geometries are apparent, and new openings for experimental investigations and theoretical analyses of planar tetracoordinate main group systems are identified. The planar conformation persists at one center in the C(BeH)3BH2 dimer, and may be identifiable in higher order clusters of ptC molecules as well.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...