Crystallization behavior and formation mechanism of dendrite Cu2O crystals†
Abstract
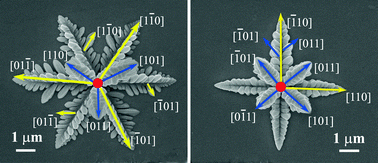
The crystal growth behavior of dendrite-like Cu2O crystals during galvanostatic electrodeposition was investigated. The deposited Cu2O particles are single-phase cuprites and their morphologies change from octahedral in a static electrolyte to dendrites in a stirred electrolyte. The morphology evolution is attributed to the enhancement of the transfer of Cu2+ and the break in the Cu2+ depletion zone around the nuclei, which promotes the deposition of Cu2+ ions along the <110> directions to form a face-centered cubic (FCC) Cu lattice under a cathodic potential. Meanwhile, O atoms diffuse into the Cu lattice to form a cuprite Cu2O lattice with a corresponding lattice expansion. Consequently, the morphology of the Cu2O crystals develops into dendrites and the growth direction of the dendritic branches are all along the <110> directions. Furthermore, the conduction type of Cu2O was also changed from p-type to n-type when the morphology of the Cu2O crystals varied from octahedral to dendrites. The mechanism of the morphology evolution in the present reaction system is helpful for controlling the morphology of the Cu2O crystals.

 Please wait while we load your content...
Please wait while we load your content...