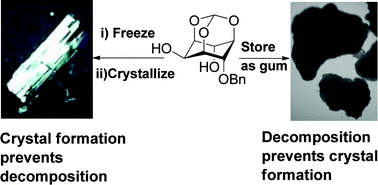
Molecular stability of racemic 4-O-benzyl-myo-inositol-1,3,5-orthoformate, an early intermediate during the synthesis of phosphoinositols, depends on the phase in which it is stored. This orthoformate is stable when stored in the crystalline form or as solution in common organic solvents. The former has eluded chemists since the preparation of this benzyl ether two decades ago. The difficulty in obtaining crystals of this orthoformate is due to the cleavage of the orthoformate moiety during storage in the gummy state. Dimorphs (form I and form II) of crystalline racemic 4-O-benzyl-myo-inositol-1,3,5-orthoformate, were obtained when the gummy sample was stored over extended periods of time. Form I crystals could be obtained consistently, by crystallization of a frozen (−20 °C) solid sample, from a solution of dichloromethane–light petroleum. The two crystal forms display dissimilar patterns of hydrogen bonding and molecular assembly in the solid-state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...