Nitrous oxide as a primary product in base-mediated β-elimination reactions of diazeniumdiolated benzylamine derivatives†
Abstract
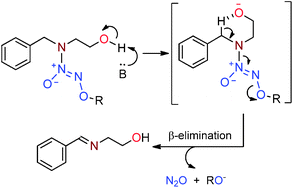
We report an unexpected β-elimination pathway by which diazeniumdiolated benzylamines of structure Bn–N(R)–N(O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OR′ undergo base-mediated fragmentation to generate N2O as the only gaseous product. The reaction is especially rapid for R =
N–OR′ undergo base-mediated fragmentation to generate N2O as the only gaseous product. The reaction is especially rapid for R = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR and R′OH.
NR and R′OH.

 Please wait while we load your content...
Please wait while we load your content...