Regiospecific β-lactam ring-opening/recyclization reactions of N-aryl-3-spirocyclic-β-lactams catalyzed by a Lewis–Brønsted acids combined superacid catalyst system: a new entry to 3-spirocyclicquinolin-4(1H)-ones†
Abstract
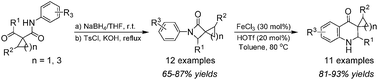
The regiospecific β-lactam ring-opening/recyclization reaction of N-aryl-3-spirocyclic-β-lactams, made by the one-pot cyclization reaction of acetoacetanilides, has been achieved for the first time using a Lewis–Brønsted acids combined superacid catalyst system, thus providing an efficient entry to 3-spirocyclicquinolin-4(1H)-ones. A mechanism involving superacid-catalysis was proposed.

 Please wait while we load your content...
Please wait while we load your content...