Applications of p-hydroxyphenacyl (pHP) and coumarin-4-ylmethyl photoremovable protecting groups†
Abstract
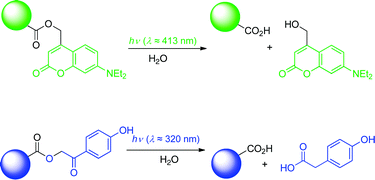
Most applications of photoremovable protecting groups have used o-nitrobenzyl compounds and their (often commercially available) derivatives that, however, have several disadvantages. The focus of this review is on applications of the more recently developed title compounds, which are especially well suited for time-resolved biochemical and physiological investigations, because they release the caged substrates in high yield within a few nanoseconds or less. Together, these two chromophores cover the action spectrum for photorelease from >700 nm to 250 nm.
- This article is part of the themed collection: Photoremovable protecting groups: development and applications

 Please wait while we load your content...
Please wait while we load your content...