Organocatalytic asymmetric tandem condensation–intramolecular rearrangement–protonation: an approach to optically active α-amino thioester derivatives†‡
Abstract
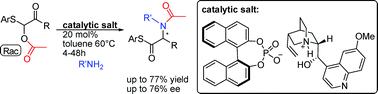
An unprecedented and conceptually novel chiral Brønsted base/Brønsted acid
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...