Suzuki–Miyaura cross-couplings of arenediazonium tetrafluoroborate salts with arylboronic acids catalyzed by aluminium hydroxide-supported palladium nanoparticles†
Abstract
Suzuki–Miyaura cross-couplings of arenediazonium salts with arylboronic acids catalyzed by highly active
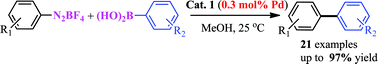

 Please wait while we load your content...
Please wait while we load your content...