Nighttime radical observations and chemistry†
Abstract
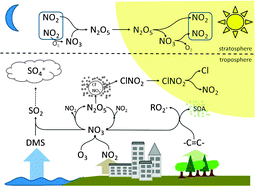
The nitrate radical, NO3, is photochemically unstable but is one of the most chemically important species in the nocturnal atmosphere. It is accompanied by the presence of dinitrogen pentoxide, N2O5, with which it is in rapid thermal equilibrium at lower tropospheric temperatures. These two nitrogen oxides participate in numerous atmospheric chemical systems. NO3 reactions with VOCs and organic sulphur species are important, or in some cases even dominant, oxidation pathways, impacting the budgets of these species and their degradation products. These oxidative reactions, together with the ozonolysis of alkenes, are also responsible for the nighttime production and cycling of OH and peroxy (HO2 + RO2) radicals. In addition, reactions of NO3 with biogenic hydrocarbons are particularly efficient and are responsible for the production of organic nitrates and secondary organic aerosol. Heterogeneous chemistry of N2O5 is one of the major processes responsible for the atmospheric removal of nitrogen oxides as well as the cycling of halogen species though the production of nitryl chloride, ClNO2. The chemistry of NO3 and N2O5 is also important to the regulation of both tropospheric and stratospheric ozone. Here we review the essential features of this atmospheric chemistry, along with field observations of NO3, N2O5, nighttime peroxy and OH radicals, and related compounds. This review builds on existing reviews of this chemistry, and encompasses field, laboratory and modelling work spanning more than three decades.
- This article is part of the themed collection: Atmospheric chemistry

 Please wait while we load your content...
Please wait while we load your content...