Tropospheric OH and HO2 radicals: field measurements and model comparisons†
Abstract
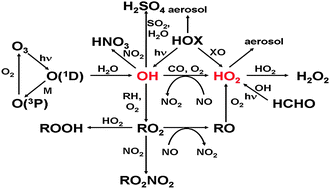
The hydroxyl radical, OH, initiates the removal of the majority of trace gases in the atmosphere, and together with the closely coupled species, the hydroperoxy radical, HO2, is intimately involved in the oxidation chemistry of the atmosphere. This critical review discusses field measurements of local concentrations of OH and HO2 radicals in the troposphere, and in particular the comparisons that have been made with numerical model calculations containing a detailed chemical mechanism. The level of agreement between field measurements of OH and HO2 concentrations and model calculations for a given location provides an indication of the degree of understanding of the underlying oxidation chemistry. We review the measurement-model comparisons for a range of different environments sampled from the ground and from aircraft, including the marine boundary layer, continental low-NOx regions influenced by biogenic emissions, the polluted urban boundary layer, and polar regions. Although good agreement is found for some environments, there are significant discrepancies which remain unexplained, a notable example being unpolluted, forested regions. OH and HO2 radicals are difficult species to measure in the troposphere, and we also review changes in detection methodology, quality assurance procedures such as instrument intercomparisons, and potential interferences.
- This article is part of the themed collection: Atmospheric chemistry

 Please wait while we load your content...
Please wait while we load your content...