Poly(methylhydrosiloxane)-supported chiral imidazolinones: new versatile, highly efficient and recyclable organocatalysts for stereoselective Diels–Alder cycloaddition reactions†‡
Abstract
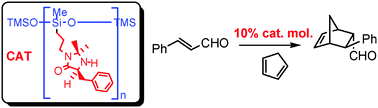
Poly(methylhydrosiloxane) (PMHS) supported organic
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...