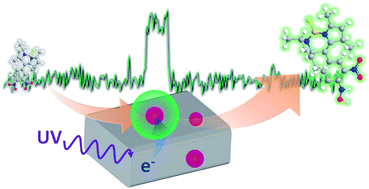
Electronic communication between the building blocks of nanocomposites is an important property that affects their functionality with regard to many optoelectronic and catalytic applications. Herein, we report a single-molecule, single-particle approach for elucidating the inherent photocatalytic activity of individual Au nanoparticle-loaded TiO2 particles using a novel redox-responsive fluorescent dye. A single-particle kinetic analysis of the fluorescence bursts emitted from the products revealed that the photocatalytic activity leading to reduction of the probe molecules is controlled by not only the substrate concentration and excitation intensity but also the Au particle size, and that these factors are intricately interrelated. Furthermore, we discovered that the stochastic photocatalytic events around the millisecond-to-second time scale showed considerable temporal and spatial heterogeneity during photoirradiation, and that they actually originate from the charging/discharging of Au nanoparticles on TiO2. Our findings represent a significant contribution to the scientific understanding of the interfacial electron transfer dynamics in composite systems, and more fundamentally, in heterogeneous (photo)chemical processes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...