Role of free space and weak interactions on geometric isomerization of stilbenes held in a molecular container†‡
Abstract
Photochemical geometric isomerization of
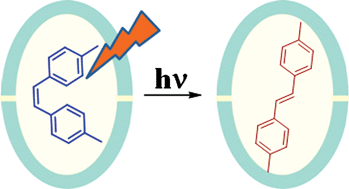
- This article is part of the themed collection: In honour of the contribution of Japanese scientists to photochemistry

 Please wait while we load your content...
Please wait while we load your content...