Photochemistry of 2-diphenylmethoxyacetophenone. Direct detection of a long-lived enol from a Norrish Type II photoreaction†‡
Abstract
Photolysis of
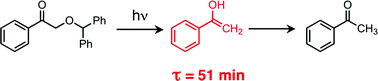
- This article is part of the themed collection: In honour of the contribution of Japanese scientists to photochemistry

 Please wait while we load your content...
Please wait while we load your content...