Synthesis of fluorinated Thomsen–Friedenreich antigens: direct deoxyfluorination of αGalNAc-threonine tert-butyl esters†
Abstract
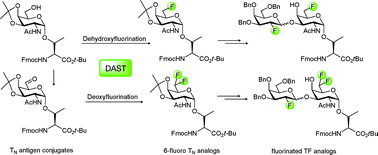
Selectively 6-fluorinated analogs of the tumor-associated TNantigen Fmoc-Thr(α-O-GalNAc)-OtBu can be efficiently prepared using DAST-mediated de(hydr)oxyfluorination reactions of preformed and orthogonally protected glycosyl amino esters without affecting the labile protecting groups and O-glycosidic linkages. The resulting mono- and difluorinated TN analogs are interesting building blocks for non-hydrolyzable mucin-type

 Please wait while we load your content...
Please wait while we load your content...