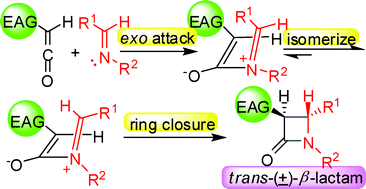
The stereoselectivity of the Staudinger reactions involving monosubstituted ketenes with electron acceptor substituents was investigated experimentally by determination of the product stereochemistry and theoretically via DFT calculations. The results indicate that imines preferentially attack the less sterically hindered exo-side of the ketenes to generate zwitterionic intermediates. Subsequently, for cyclic imines, the intermediates undergo a conrotatory ring closure directly to produce β-lactams, while for linear imines, the imine moiety of the intermediates isomerizes to more stable intermediates, which further undergo a conrotatory ring closure to afford trans-β-lactams. The steric hindrance and the isomerization, rather than the torquoelectronic effect, play crucial roles in controlling the stereoselectivity in the practical Staudinger reactions involving monosubstituted ketenes with electron acceptor substituents, although the unaccessible borylketene with a powerful electron acceptor group controls the stereoselectivity torquoelectronically, in theory.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...