Intramolecular Diels–Alder chemistry of 4-vinylimidazoles†
Abstract
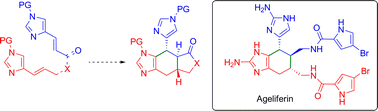
An investigation of 4-vinylimidazoles as diene components in the intramolecular Diels–Alder reaction is described. In the course of these studies several parameters affecting the cycloaddition were evaluated including the nature of the

 Please wait while we load your content...
Please wait while we load your content...