Heteropoly acid-catalyzed microwave-assisted three-component aza-Diels–Alder cyclizations: diastereoselective synthesis of potential drug candidates for Alzheimer's disease
Abstract
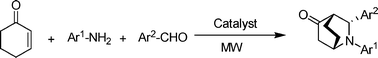
A highly diastereoselective microwave-assisted three component synthesis of azabicyclo[2.2.2]octan-5-ones by a
 Please wait while we load your content...
Please wait while we load your content...