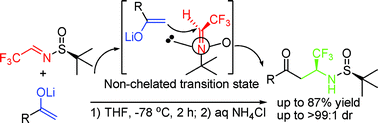
A facile process for the asymmetric synthesis of β-trifluoromethylated β-amino ketonesvia addition of ketone enolates to sulfinylimine†‡
Abstract
An efficient method for the asymmetric synthesis of β-trifluoromethylated β-amino ketonesvia addition of
 Please wait while we load your content...
Please wait while we load your content...