Diphenylbutylpiperidine-based cell autophagy inducers: Design, synthesis and SAR studies†
Abstract
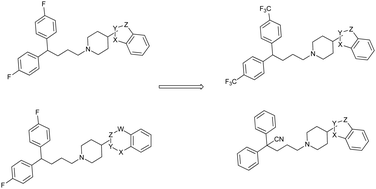
A novel series of diphenylbutylpiperidine derivatives in which a benzo-five or six membered heterocycle was linked at the 4-position of the piperidine moiety were designed and synthesized. Structure–activity relationship (SAR) studies of these compounds indicated that some molecules show promising autophagy inducing activity. Replacement of the fluorine atom by a CF3 group on the diphenyl part resulted in significant enhancement of the autophagy inducing effect. In the addition, a group of diphenylpentyl
 Please wait while we load your content...
Please wait while we load your content...