Electrochemistry of Os(2,2′-bpy)2ClPyCH2NHCOPh tethered to Au electrodes by S–Au and C–Au junctions†
Abstract
Osmium pyridine-
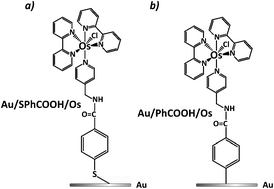
- This article is part of the themed collection: Interfacial processes and mechanisms
* Corresponding authors
a
INQUIMAE, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, CONICET Pabellón 2, Ciudad Universitaria, 1428 Buenos Aires, Argentina
E-mail:
calvo@qi.fcen.uba.ar
Fax: 541145763341
Tel: 541145763378
b Centro Atómico Bariloche and Instituto Balseiro, Comisión Nacional de Energía Atómica, 8400 S. C. de Bariloche, Río Negro, Argentina
c INIFTA, Universidad Nacional de La Plata, CONICET, Sucursal 4 Casilla de Correo 16, 1900 La Plata, Argentina
Osmium pyridine-

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. M. Ricci, N. Tognalli, E. de la Llave, C. Vericat, L. P. Méndez De Leo, F. J. Williams, D. Scherlis, R. Salvarezza and E. J. Calvo, Phys. Chem. Chem. Phys., 2011, 13, 5336 DOI: 10.1039/C0CP02409K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
