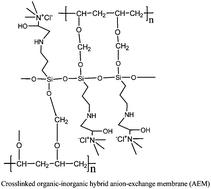
Alkaline membranes also termed as anion exchange membranes (AEMs) have recently become important materials for electrochemical technology, alkaline fuel cells, and electrolyzers. We report a simple synthesis procedure for an anion-exchange silica precursor (AESP) by epoxide ring opening reaction using 3-aminopropyltriethoxysilane (APTEOS) and glycidyltrimethylammonium chloride (GDTMAC). The AESP-poly(vinyl alcohol) (PVA) organic-inorganic hybrid AEM was prepared by the sol–gel method in acidic medium followed by chemical crosslinking of –OH groups via formal reaction. The reported method is a “green” alternative for the production of AEM without the use of any solvent residues or hazardous chemicals. This simplified procedure in aqueous media avoids the use of chloromethyl methyl ether (CME), a carcinogen and harmful to human health. These AEMs (especially AEM-70) were designed to possess all the required properties of a highly anion conductive membrane such as high water uptake (67.3%), ion-exchange capacity (1.36 mequiv g−1), and permselectivity (0.94), along with reasonable conductivity (7.61 mS cm−1) due to quaternary ammonium group functionality. Electroosmotic studies revealed quite low mass drag and equivalent pore radius (2.31–5.28 A°) of the membrane, which are also desirable properties of an efficient AEM. Electrodialytic performance of the AEM-70 membrane revealed its suitability for applications in electro-membrane processes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...