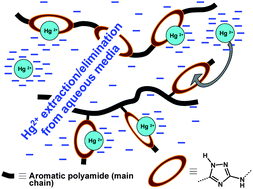
Novel aromatic polyamides with main chain and pendant 1,2,4-triazole moieties and their application to the extraction/elimination of mercury cations from aqueous media
Abstract
This work describes five novel aromatic polyamides and copolyamides containing the

 Please wait while we load your content...
Please wait while we load your content...