Manganese catalyzed cis-dihydroxylation of electron deficient alkenes with H2O2Electronic supplementary information (ESI) available: Full details for reactions in Tables 1 and 2 with characterisation and spectra. See DOI: 10.1039/c0ob00102c
Abstract
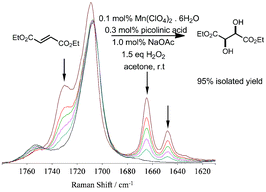
A practical method for the multigram scale selective cis-dihydroxylation of electron deficient alkenes such as diethyl fumarate and N-alkyl and N-aryl-maleimides using H2O2 is described. High turnovers (>1000) can be achieved with this efficient manganese based catalyst system, prepared in situ from a manganese salt, pyridine-2-carboxylic acid, a ketone and a base, under ambient conditions. Under optimized conditions, for diethyl fumarate at least 1000 turnovers could be achieved with only 1.5 equiv. of H2O2 with d/l-diethyl tartrate (cis-diol product) as the sole product. For electron rich alkenes, such as cis-cyclooctene, this catalyst provides for efficient epoxidation.
 Please wait while we load your content...
Please wait while we load your content...