Direct catalytic asymmetric synthesis of highly functionalized tetronic acids/tetrahydro-isobenzofuran-1,5-diones via combination of cascade three-component reductive alkylations and Michael-aldol reactions†
Abstract
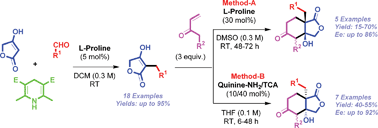
A practical and sustainable chemical process for the synthesis of highly substituted tetrahydro-isobenzofuran-1,5-diones was achieved for the first time through asymmetric cascade Michael-aldol reaction of 4-hydroxy-3-alkyl-5H-furan-2-ones with alkyl vinyl ketones in the presence of a

 Please wait while we load your content...
Please wait while we load your content...