Conformational analysis of the natural iron chelatormyo-inositol 1,2,3-trisphosphate using a pyrene-based fluorescent mimic†
Abstract
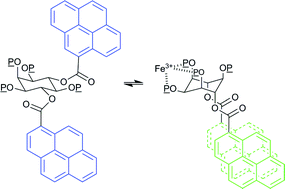
myo-Inositol phosphates possessing the
* Corresponding authors
a
School of Pharmacy & Pharmaceutical Sciences, University of Manchester, Oxford Road, Manchester, UK
E-mail:
sally.freeman@manchester.ac.uk
Fax: +44 161 275 2396
Tel: +44 161 275 2366
b School of Life and Health Sciences, Aston University, Birmingham, UK
c School of Chemistry, The University of Nottingham, University Park, Nottingham, UK
d Cátedra de Química Inorgánica, Departamento Estrella Campos, Facultad de Química, Universidad de la República, Montevideo, Uruguay
e Rolf Luft Research Center for Diabetes and Endocrinology, Karolinska Institutet, Stockholm, Sweden
myo-Inositol phosphates possessing the

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
D. Mansell, N. Rattray, L. L. Etchells, C. H. Schwalbe, A. J. Blake, J. Torres, C. Kremer, E. V. Bichenkova, C. J. Barker and S. Freeman, Org. Biomol. Chem., 2010, 8, 2850 DOI: 10.1039/C001078B
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
