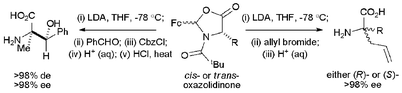
Treatment of a range of cis- and trans-2-ferrocenyl-3-pivaloyl-4-alkyl-1,3-oxazolidin-5-ones with LDA followed by the addition of allyl bromide promotes highly stereoselective allylation (>98% de) at the 4-position of the oxazolidinone ring anti to the stereodirecting 2-ferrocenyl group. Hydrolysis of the resultant 4,4-disubstituted oxazolidinones (>98% de) yields enantiomeric (R)- and (S)-2-alkyl-2-aminopent-4-enoic acids in high ee. Furthermore, the aldol reaction of the lithium enolate of cis-2-ferrocenyl-3-pivaloyl-4-methyl-1,3-oxazolidin-5-one with benzaldehyde followed by in situ O-protection affords O-protected aldol products in >98% de, with hydrolysis affording (2R,3S)-2-amino-2-methyl-3-hydroxy-3-phenylpropanoic acid in >98% de.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...