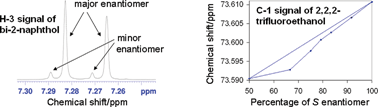
Molecular modeling of the homo- and heterochiral dimeric self-associates of the enantiomers of 1,1′-bi-2-naphthol and 1-phenyl-2,2,2-trifluoroethanol in solution has been performed in order to understand their NMR behavior and in light of the phenomenon of “enantiomer self-disproportionation on achiral-phase chromatography” (ESDAC). For 1,1′-bi-2-naphthol in C6D6, distinct NMR signals for each enantiomer arise for some spins in non-racemic mixtures—the phenomenon of self-induced diastereomeric anisochronism (SIDA). The linear divergence of these split signals across an enantiomeric titration (a series of samples in which the percentage of one enantiomer is varied from 50–100% whilst maintaining a constant total concentration), as well as the near linear migration of certain signals in CDCl3 across a similar enantiomeric titration, where signals were not observed to be split, is consistent with the calculated small energy differences between the homo- and heterochiral associates. For an enantiomeric titration of 1-phenyl-2,2,2-trifluoroethanol in n-hexane, NMR signals also remained unsplit but the noticeable migration of some revealed a skew indicative of a preference for the heterochiral associate. This was duly reflected in the calculations which provided a ΔG value favoring the heterochiral associate by 2.4 kJ mol−1. The relevance of these results to evaluating the likely occurrence of ESDAC is considered.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...