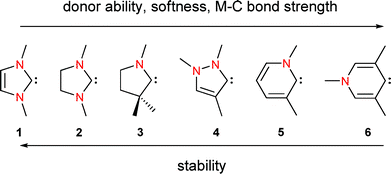
Electronic structure, thermodynamic stability and ligand properties in LRh(CO)2Cl complexes of a series of N-heterocyclic carbenes (NHCs) were studied at the DFT level. The systems under study are: imidazolin-2-ylidene (1), imidazolidin-2-ylidene (2), cyclic(alkyl)(amino)carbene (CAAC, 3), pyrazolin-3-ylidene (4), pyridin-2-ylidene (5), and pyridin-4-ylidene (6). The main structural feature influencing the properties of these species is the number of nitrogen atoms at the ylidene carbon. A decrease of the number of nitrogen atoms on the one hand leads to an increase in donor ability and ligand-to-metal bond strength, but lowers the stability of the NHC on the other hand. The number of nitrogen atoms can be taken as a key parameter for the classification of carbenes into 2N-NHC, 1N-NHC and r-NHC (r = remote).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...