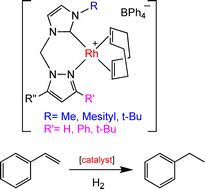
A series of bidentate 1-(1-pyrazolylmethyl)-substituted NHC ligands (13a–c, 14a–c and 15a–c) were synthesised with substituents of varying steric bulk incorporated adjacent to the donor atoms. These ligands were coordinated to rhodium(I) to give a series of complexes of the general formula [Rh(L)(COD)]BPh4 (where L = a mixed-donor pyrazolyl-NHC ligand and COD = 1,5-cyclooctadiene). The solid state structures of [Rh(13b)(COD)]BPh4 (16b), [Rh(13c)(COD)]BPh4 (16c), [Rh(14a)(COD)]BPh4 (17a), [Rh(14b)(COD)]BPh4 (17b), [Rh(15a)2(COD)]BPh4 (18a), and [Rh(15b)(COD)]BPh4 (18b) were determined by single crystal X-ray diffraction. The complex [Rh(15a)2(COD)]BPh4 (18a) is unusual in that two of the pyrazolyl-NHC ligands (15a) are coordinated to the metal through the NHC donor instead of one ligand forming the expected chelate. These complexes (with the exception of 18a) were found to be effective catalysts for the hydrogenation of styrene. The catalytic activity was correlated with complex structure, and it was found that the greater the steric bulk of the metal bound ligand, the slower the rate of the hydrogenation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...