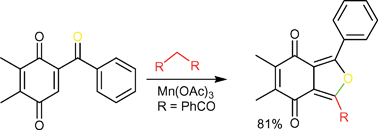
A manganese(III)-mediated reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds that produces benzo[c]furan-4,7-diones and anthracene-1,4-diones with high chemoselectivity is described. With ethyl butyrylacetate, by changing the solvent, benzo[c]furan-4,7-diones and anthracene-1,4-diones can be generated in high chemoselectivities. With ethyl benzoylacetate, N,N-dimethyl acetoacetamide and 1,3-diones, benzo[c]furan-4,7-diones were produced effectively with high selectivity. With 2-alkyl-5-benzoyl-1,4-benzoquinones, the regioselectivity of this reaction was also studied and the corresponding benzo[c]furan-4,7-dione and anthracene-1,4-dione derivatives were obtained in high regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...