Thio-arylglycosides with various aglyconpara-substituents: a probe for studying chemical glycosylation reactions†
Abstract
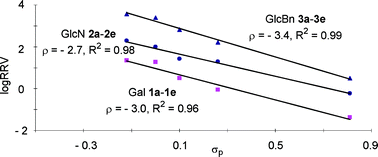
Three series of thioglycosyl donors differing only in their respective aglycon substituents within each series have been prepared as representatives of typical

 Please wait while we load your content...
Please wait while we load your content...