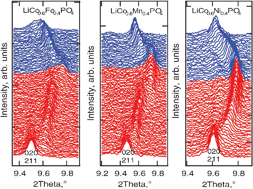
The electrochemical delithiation of LiCo0.6M0.4PO4 phosphates (M = Mn, Fe, Ni) was studied by in situsynchrotron diffraction. In all three metallophosphates the oxidation–reduction of 3d-elements proceed via two-phase mechanisms leading to two-phase regions, corresponding to the Co2+/Co3+ and M2+/M3+ reactions. The Ni2+/Ni3+ reaction was not revealed, neither by the potentiostatic intermittent titration technique (PITT) nor by diffraction. In the two-phase reaction, the olivine-like structure of the cathode remains preserved, which is characteristic of this type of materials. Pronounced solid-solution domains were observed during both lithium extraction and insertion. The thermal stability of the charged cathodes is limited by the presence of Co3+ and its intrinsic instability in these compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...