Hydrogen adsorption on Pd-containing Au(111) bimetallic surfaces
Abstract
Configurations of different Pd-containing Au(111) bimetallic surfaces with Pd substituents varying from one to three atoms have been studied using density functional theory. The stability of the so-formed Pd monomers, dimers or trimers in the surface and subsurface layers of a Au(111)-(3 × 3) unit cell and their influence on the adsorption of hydrogen have been investigated. We find that before hydrogen
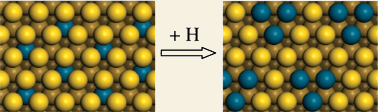
- This article is part of the themed collection: Physical chemistry of solids - The science behind materials engineering

 Please wait while we load your content...
Please wait while we load your content...