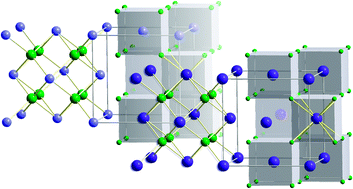
A highly conductive ternary fluoride with mixed cations is prepared by joint high-energy ball milling of cubic BaF2 and CaF2 in the ratio 0.4 : 0.6. The sample produced at room temperature consists of a nanocrystalline, defect-rich mixed (Ba,Ca)F2 phase with retained cubic symmetry as well as of single-phase CaF2 particles. The anion conductivity of the mixed phase, which decomposes at higher temperature (770 K) into BaF2 and CaF2, exceeds that of single-phase nanocrystalline BaF2 by two and that of CaF2 by four orders of magnitude. In turn, these conductivities are each greater by about two orders than those of the respective microcrystalline counterparts. Structural features of the samples are characterized by X-ray diffraction, TEM and 19F MAS NMR spectroscopy. Static 19F NMR spectra confirm the unexpectedly high anion conductivity probed by impedance spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...