B-Site cation diffusivity of Mn and Cr in perovskite-type LaMnO3 with cation-deficit nonstoichiometry
Abstract
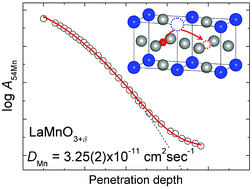
The Mn
- This article is part of the themed collection: Physical chemistry of solids - The science behind materials engineering

 Please wait while we load your content...
Please wait while we load your content...