Palladium-catalyzed aryl halide carbonylation–intramolecular O-enolate acylation: efficient isocoumarin synthesis, including the synthesis of thunberginol A†‡
Abstract
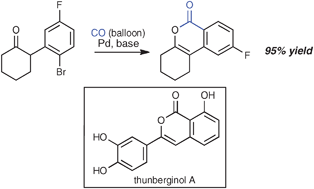
Exposure of a series of α-(o-haloaryl)-substituted
- This article is part of the themed collection: Selective Catalysis for Organic Synthesis

 Please wait while we load your content...
Please wait while we load your content...