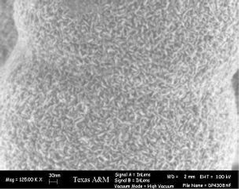
The past decade has witnessed an exponential growth of metal organic framework compounds (MOFs). The defining character of these compounds is their porosity. However, in many cases no effort was made to show evidence that a stable porous structure has been achieved and that the pores may be accessed. In the present paper we describe recent work on porous pillared zirconium diphosphonates, and the newer and in many respects different characteristics of tin(IV) phosphonates. The Sn(IV) monophosphonates form spherical globules that exhibit very high surface areas. The surface area arises from their nano-sized particles that pack in a “house of cards” arrangement. Also, it is shown that the 1,4-monophenyldiphosphonic acid forms highly porous (250–400 m2 g−1) materials with Sn(IV) when prepared in alcohol–water media. This is not the case with analogous Zr(IV) compounds. The many variations in the syntheses of both the zirconium and tin aryl- and alkyldiphosphonate pillars and their combinations with spacers such as methyl- and monophenylphosphonic acid have created a variety of highly porous materials that are stable to 400 °C in air, highly stable in acid media, do not collapse when de-solvated, and can be post and presynthesis altered to include functional groups. Several new directions taken by other researchers are also described. However, it is emphasized in this presentation that the cross-linked compounds form particles that precipitate rapidly into nanoparticles that exhibit only short range order. Therefore, they differ from the more conventional MOFs in that they are not amenable to structure solution by X-ray or neutron diffraction techniques. Rather, they must be understood on the basis of modeling and indirect data from EM, NMR, and additional spectroscopic and textural studies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...