The role of “external” lone pairs in the chemical bonding of bare post-transition element clusters: the Wade–Mingos rules versus the jellium model
Abstract
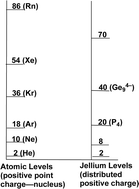
The jellium sphere model of a volume of electrons, counterbalanced by a positive charge throughout the sphere, leads to an energy level sequence corresponding to special stabilities of bare post-transition element clusters with 20 valence electrons such as the known P4 and clusters with 40 valence electrons such as the known Ge94−, Ni@In1010−, and In117−. In this model the otherwise “external” lone pairs on the vertex atoms participate at least indirectly in the skeletal bonding. Furthermore, this model predicts the most favorable polyhedra and electron counts in some cases to be quite different than those predicted by the Wade–Mingos rules of polyhedral borane chemistry.

 Please wait while we load your content...
Please wait while we load your content...