Organocatalytic asymmetric aldol reaction in the presence of water
Abstract
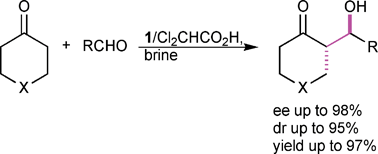
Water was found to be a suitable solvent for the L-prolinethioamide catalysed aldol reaction of various cyclic ketones with aromatic aldehydes. Treatment of 4-nitrobenzaldehyde with as little as 1.2 equiv. of cyclohexanone in the presence of the protonated catalyst 1–TFA, afforded aldol products in high yields (up to 97%) with high diastereo- and enantioselectivity (up to >5 : 95 dr and 98% ee). The use of a high excess of ketone was avoided by conducting the aldol addition in the presence of water. Furthermore, different ‘salting-out’ and ‘salting-in’ salts were investigated and it was proven that the rate of acceleration and the stereochemical outcome of the reaction are affected by hydrophobic aggregation. Scope and limitation studies revealed that electron deficient aldehydes afforded aldol products with high stereoselectivity in the presence of 1–Cl2CHCO2H. It was shown that various cyclic ketones, under the conditions found, gave aldol products with fair yields, even if they are used in substoichiometric amounts (1.2 to 2.0 equiv.).

 Please wait while we load your content...
Please wait while we load your content...