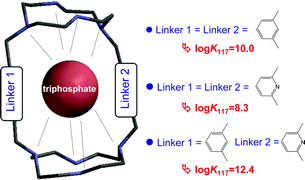
The host–guest interaction between orthophosphate, pyrophosphate and triphosphate anions and three cyclen-based macrotricyclic ligands was investigated by potentiometric measurements and NMR spectroscopy. The ligands differ from one another by the nature of their spacers, which are 1,3-dimethylbenzene (TMC), 2,6-dimethylpyridine (TPyC) or a combination of the two (TMPyC). In aqueous solution, each ligand gave protonated species that further formed ternary complexes after binding with anions; these complexes were analyzed as a result of hydrogen bond formation and coulombic attraction between the organic host and the inorganic guest. The equilibrium constants found for all the detected species are reported and the selectivity, illustrated with species distribution diagrams, is discussed. The results unambiguously showed that the ligand possessing a single supplementary anchoring site (the pyridinyl spacer) exhibited the greatest affinity for the phosphate species in a large p[H] range.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...