Nucleophilic 5-endo-trig cyclizations of N-homoallylic sulfonamides: a facile method for the construction of pyrrolidine rings†
Abstract
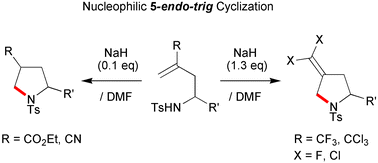
Normally disfavored 5-endo-trig cyclizations proceed in N-homoallylsulfonamides bearing a CF3, CCl3, CO2Et or CN group at the C-3 position, via an intramolecular SN2′-type or addition reaction to construct pyrrolidine rings, even though the system allows a more favorable 5-exo-trig pathway.

 Please wait while we load your content...
Please wait while we load your content...