The design and synthesis of inhibitors of pantothenate synthetase†
Abstract
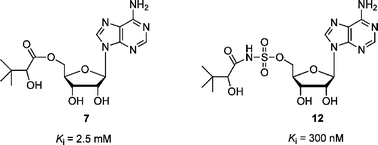
Pantothenate synthetase catalyses the ATP-dependent condensation of D-pantoate and β-alanine to form pantothenate. Ten analogues of the reaction intermediate pantoyl adenylate, in which the phosphodiester is replaced by either an ester or sulfamoyl group, were designed as potential inhibitors of the enzyme. The esters were all modest competitive inhibitors, the sulfamoyls were more potent, consistent with their closer structural similarity to the pantoyl adenylate intermediate.
- This article is part of the themed collection: In memory of Chris Abell

 Please wait while we load your content...
Please wait while we load your content...