Synthesis of α-amino acids by reaction of aziridine-2-carboxylic acids with carbon nucleophiles†
Abstract
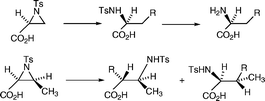
A variety of homochiral α-amino acids have been prepared in good yield via regioselective reaction of higher order cuprates with (2S)-N-para-toluenesulfonylaziridine-2-carboxylic acid 4. The reaction was much less regioselective and low yielding when higher order cuprates were reacted with the more hindered aziridine carboxylic acid 30, the principal products being protected β-amino acids. Reaction of lithium trimethylsilylacetylide with the aziridine acid 30, however, gave a protected α-amino acid which was converted to the protected isoleucine ester 37.

 Please wait while we load your content...
Please wait while we load your content...