Synthesis of naturally occurring iminosugars from d-fructose by the use of a zinc-mediated fragmentation reaction†
Abstract
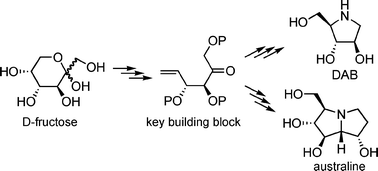
A short synthesis of 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) and a formal synthesis of australine are described. In both cases, D-fructose is employed as the starting material and converted into a protected methyl 6-deoxy-6-iodo-furanoside. Zinc-mediated fragmentation produces an unsaturated ketone which serves as a key building block for both syntheses. Ozonolysis, reductive amination with benzylamine and deprotection affords 1,4-dideoxy-1,4-imino-D-arabinitol in only 7 steps and 11% overall yield from D-fructose. Alternatively, reductive amination with homoallylamine, ring-closing metathesis and protecting group manipulations give rise to an intermediate which can be converted into australine in 3 steps. The intermediate is prepared by two different strategies both of which use a total of 9 steps. The first strategy utilizes benzyl ethers for protection of fructose while the second and more effective strategy employs an isopropylidene acetal.

 Please wait while we load your content...
Please wait while we load your content...