Polypyridyl ruthenium complexes as coating agent for the formation of gold and silver nanocomposites in different media. Preliminary luminescence and electrochemical studies†‡
Abstract
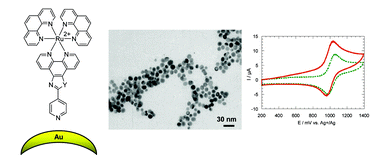
Three polypyridyl ruthenium complexes, [(phen)2Ru(2,2′-p-phenylene-bis(imidazo[4,5-f][1,10])phenanthroline)](X)2 (1·X2), [(phen)2Ru((4-pyridine)oxazo[4,5-f][1,10]phenanthroline)](X)2 (2·X2) and [(phen)2Ru((3-thiophene)imidazo[4,5-f][1,10]phenanthroline)](X)2 (3·X2), X− = PF6− or Cl−, have been used to functionalize and stabilize gold and silver nanoparticles in aqueous and non aqueous (acetonitrile) media. Direct interaction between the metallic nanoparticles and the ruthenium complexes was ensured by bidentate chelating phenanthroline (1), monodentate pyridine (2) or monodentate thiophene (3) groups. The influence of several parameters was studied in order to control the size, shape and stability of the nanocomposites thus formed: the nature of the metallic nanoparticles, the nature of the coating ruthenium complex, the ratio R = [Mn+]/[Ru2+] (M = Ag or Au) and the solvent. The colloidal solutions have been characterized by UV-Vis spectroscopy, transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). Preliminary electrochemical and luminescence measurements have shown the influence of the metallic nanoparticle on the properties of the ruthenium complexes.

 Please wait while we load your content...
Please wait while we load your content...