Multi-mechanism linear free energy relationships and isoequilibrium or isokinetic temperatures
Abstract
The equations that describe the temperature dependence of the various terms in multi-mechanism linear free energy relationships (MMLFERs) are outlined with respect to reactions of organometallics involving P-donors. Each MMLFER includes a term defining the free energy of a standard reaction characterized by a chosen standard P-donor. Other terms quantify the various mechanisms by which the free energy of the standard reaction is modified by the participation of other P-donors that have different electronic and steric properties. Several different electronic terms can be involved. The temperature dependence of the modifying terms can provide measurements of the separate enthalpic and entropic contributions to the modifying processes. For MMLFERs that involve equilibrium or rate constants, each modifying term is associated with its own isoequilibrium or isokinetic temperature (IET or IKT) which defines the temperature at which each term is reduced to zero. These temperatures are provided by the ratios of the enthalpic and entropic contributions and it is suggested that they have little or no theoretical significance in themselves, although they can of course be of practical importance. There can be no meaningful single IET or IKT for any single series of reactions that show MMLFERs. The equations defining the temperature dependence of the modifying terms are applied to an extensive set of published
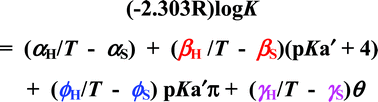

 Please wait while we load your content...
Please wait while we load your content...