Synthesis and characterization of manganese(ii) and iron(iii) d5 tripodal imidazole complexes. Effect of oxidation state, protonation state and ligand conformation on coordination number and spin state
Abstract
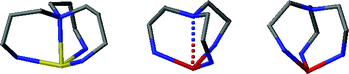
The 1 : 3 Schiff base condensates of tris(2-aminoethyl)amine (tren) or tris(3-aminopropyl)amine (trpn) with 4-methyl-5-imidazolecarboxaldehyde, H3L1 and H3L2, respectively, were generated in situ and used to prepare complexes with manganese(II) and iron(III). The resultant complexes, [MnH3L1](ClO4)2, [MnH3L1](ClO4)2·EtOH·H2O, [MnH3L2](ClO4)2, [FeH3L1](ClO4)3·1.5(EtOH) and [FeHL1](I3) 0.525(I)0.475·2.625H2O, have been characterized by EA, IR, ES MS, variable temperature magnetic susceptibility, X-ray crystallography, and Mössbauer spectroscopy for the iron complexes. The three manganese(II) complexes are high spin with [MnH3L2](ClO4)2 exhibiting coordination number seven while the others are six coordinate. [FeH3L1](ClO4)3·1.5(EtOH) has two iron sites, a seven coordinate and a pseudo seven coordinate site. The complex is high spin at room temperature but exhibits a magnetic moment that decreases with temperature corresponding to conversion of one of the sites to low spin. [FeHL1](I3) 0.525(I)0.475·2.625H2O is low spin even at room temperature. In the present complexes the apical nitrogen atom, Nap, of the tripodal ligand is pyramidal and directed toward the metal atom. The data show that the M–Nap distance decreases as the oxidation state of the metal increases, as the number of bound imidazole protons on the ligand increases, and as the number of carbon atoms in the backbone of the ligand (tren vs. trpn) increases. In a limiting sense, short M–Nap distances result in high spin seven coordinate mono capped octahedral complexes and long M–Nap distances result in low spin six coordinate octahedral complexes.

 Please wait while we load your content...
Please wait while we load your content...